- Home
- Healthcare
-
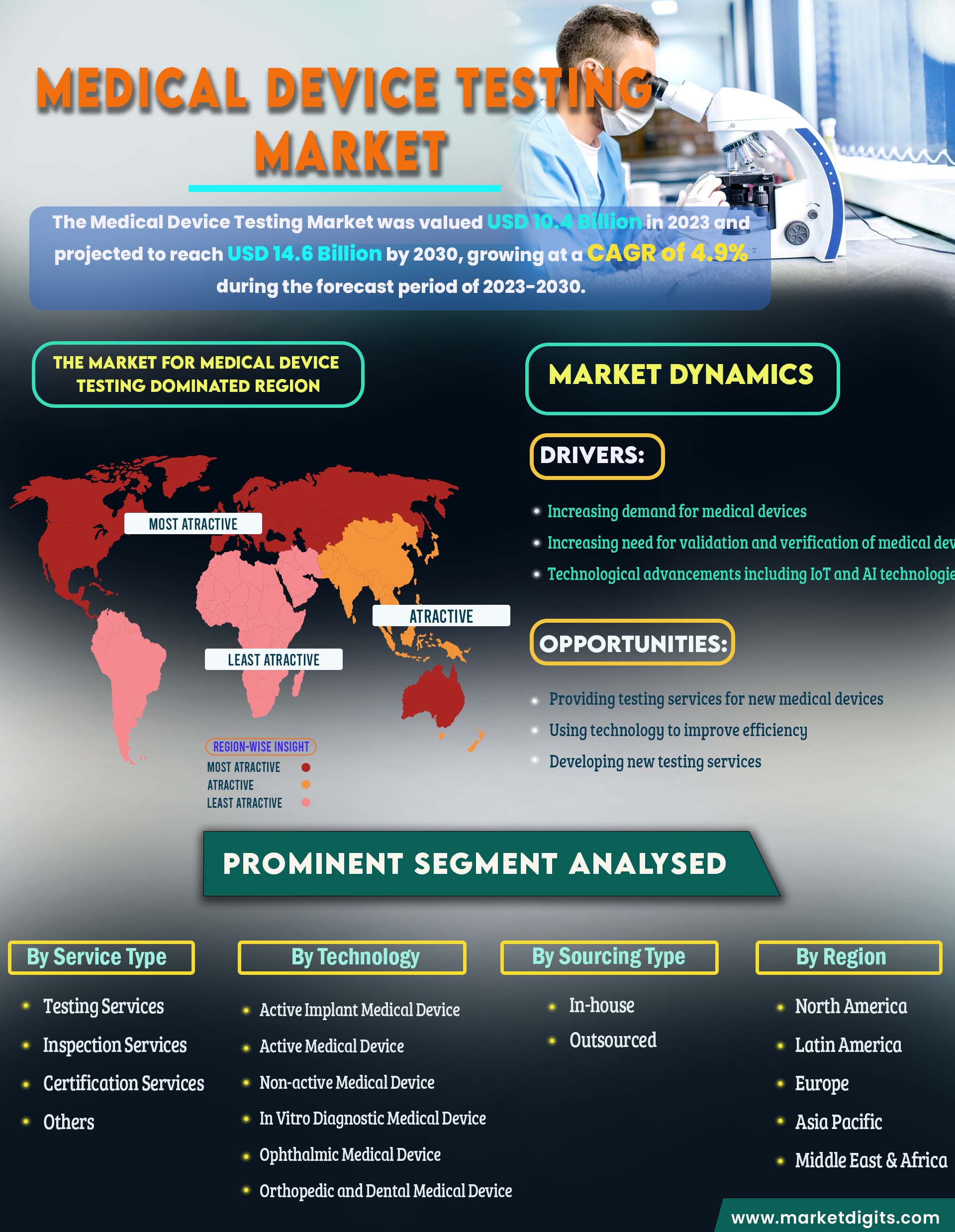
Medical Device Testing Market

Medical Device Testing Market , by Service Type , Sourcing Type (In-house and Outsourced), Phase (Preclinical and Clinical), Device Class (Class I, Class II and Class III), Technology (Active Implant Medical Device, Active Medical Device) and Region - Partner & Customer Ecosystem (Product Services, Proposition & Key Features) Competitive Index & Regional Footprints by MarketDigits - Forecast 2024-2032
Industry : Healthcare | Pages : 184 Pages | Published On : Apr 2024
Market Overview
The evaluation of medical devices, known as medical device testing, involves a comprehensive process to ensure their safety, effectiveness, and performance align with regulatory standards. This includes a series of tests, experiments, and evaluations that cover various aspects such as design, functionality, durability, sterility, biocompatibility, electrical safety, and usability. The demand for testing has increased due to the expanding presence of medical device manufacturers in emerging markets, necessitating compliance with diverse regulatory environments. The growing complexity of devices, including those with multiple components and integrated software, requires intricate testing protocols, contributing to market growth. Competitive pressures driving companies to attain certifications and standards for a competitive edge further support market expansion. Additionally, widespread adoption of testing as a proactive risk management strategy to prevent high litigation costs from device malfunctions boosts market growth. The rise in global healthcare expenditure, leading to increased demand for quality-assured medical devices, is also a positive factor influencing market growth.
Major vendors in the global Medical Device Testing market: BSI Group, Dekra Testing and Certification GmbH, Eurofins Scientific, Institute for testing and Certification Inc., Intertek Group PLC, SGS SA, TUV Rheinland, UL LLC, Bureau Veritas, Element Materials Technology, Gateway Analytical LLC, Medistri SA, Pace Analytical Services LLC, WuXi AppTec and Others.
The global expansion of the healthcare industry
The expanding healthcare industry on a global scale presents a significant opportunity for various players in the medical device testing market. With improved healthcare access worldwide, especially in emerging markets, there is a rising demand for advanced medical devices. This demand is driven by factors such as population growth, increasing disposable incomes, and a growing awareness of healthcare and wellness. Consequently, there is a need for reliable testing services to ensure that these devices meet stringent regulatory standards in terms of safety, effectiveness, and compliance. Testing facilities and services play a crucial role in supporting the worldwide expansion of the healthcare industry by offering assurance to manufacturers, healthcare providers, and patients. This, in turn, contributes to the overall growth and credibility of the global medical device sector.
Market Dynamics
Drivers:
- Increasing demand for medical devices
- Increasing need for validation and verification of medical devices
- Technological advancements including IoT and AI technologies
Opportunities:
- Providing testing services for new medical devices
- Using technology to improve efficiency
- Developing new testing services
Connectivity of mobile devices with medical equipment
The integration of mobile technology with medical devices is transforming the healthcare landscape. Dedicated applications and connectivity enable seamless interactions between mobile devices and medical equipment, empowering individuals to monitor and manage their health on the move. This integration enhances accessibility and enables timely interventions, leading to improved health outcomes. The rapid progress in wireless technologies and sensor-based devices has ushered in a new era of mobile medical devices, significantly improving the quality of life for patients while lowering healthcare costs for both providers and individuals. The compact size and networking capabilities of these mobile medical devices provide substantial benefits, allowing patients to make fewer hospital visits and affording healthcare providers the advantage of reduced administrative overheads and care expenses.
The market for Medical Device Testing is dominated by North America.
In 2022, North America emerged as the dominant force in the market. In North America, the stringent regulatory landscape is compelling companies to heavily invest in thorough evaluation processes. This strict regulatory framework naturally boosts the demand for advanced testing services. Moreover, the region is a hub for technological innovation, hosting numerous high-tech companies, research institutions, and startups dedicated to medical devices. The rapid development of new technologies requires equally advanced testing services, fostering a thriving market. Additionally, North America contributes significantly to global healthcare spending, and the substantial investments in healthcare infrastructure and medical technology contribute to the need for extensive and specialized medical device testing.
The Asia-Pacific, rapid economic growth translates into increased healthcare expenditure, driving the demand for a diverse range of medical devices and, consequently, their testing. The region is also experiencing substantial expansion in healthcare infrastructure, including hospitals and clinics, leading to a growing demand for certified medical devices and testing services. Additionally, various policies implemented by regional governments to strengthen medical device regulations are propelling market growth.
The Class III Segment is Anticipated to Hold the Largest Market Share During the Forecast Period
Based on device class, the market is segmented into Class I, Class II, and Class III. In 2022, the market was predominantly led by the Class III segment and is anticipated to maintain the largest share in Medical Device Testing throughout the forecast period. Regulatory bodies, notably the U.S. FDA, typically subject Class III devices to more rigorous testing and certification requirements. The regulatory emphasis on stringent testing ensures that manufacturers of these devices make significant investments in testing services. Compared to Class I devices, Class III devices tend to have a larger market size in Medical Device Testing. This financial incentive motivates medical device manufacturers to allocate resources for comprehensive testing, safeguarding their investments. Class III encompasses many breakthrough and innovative medical devices, including implantable devices, advanced diagnostic equipment, and complex surgical instruments, which necessitate cutting-edge testing methods to assess their safety and efficacy.
Segmentations Analysis of Medical Device Testing Market: -
- By Service Type
- Testing Services
- Functional Testing
- Performance Testing
- Safety Testing
- Biocompatibility Testing
- Usability Testing
- Software Validation and Verification
- Environmental Testing
- Inspection Services
- Certification Services
- Others
- Inspection Services
- Certification Services
- Others
- Testing Services
- By Sourcing Type
- In-house
- Outsourced
- By Phase
- Preclinical
- Clinical
- By Device Class
- Class I
- Class II
- Class III
- By Technology
- Active Implant Medical Device
- Active Medical Device
- Non-active Medical Device
- In Vitro Diagnostic Medical Device
- Ophthalmic Medical Device
- Orthopedic and Dental Medical Device
- Others
- By Region
- North America
- US
- Canada
- Latin America
- Brazil
- Mexico
- Argentina
- Colombia
- Chile
- Peru
- Rest of Latin America
- Europe
- Germany
- France
- Italy
- Spain
- U.K.
- BENELUX
- CIS & Russia
- Nordics
- Austria
- Poland
- Rest of Europe
- Asia Pacific
- China
- Japan
- South Korea
- India
- Thailand
- Indonesia
- Malaysia
- Vietnam
- Australia & New Zealand
- Rest of Asia Pacific
- Middle East & Africa
- Saudi Arabia
- UAE
- South Africa
- Nigeria
- Egypt
- Israel
- Turkey
- Rest of MEA
- North America
Recent Developments
- In September 2023, Bureau Veritas has successfully acquired Galbraith Laboratories Inc., recognized for its advanced analytical solutions in the healthcare sector. This strategic acquisition strengthens Bureau Veritas' position in the supply chains of Consumer Healthcare and Industrial Chemicals, providing robust support for research, development, and product testing. The specialized knowledge from Galbraith Laboratories will enrich Bureau Veritas' service offerings, with a particular focus on enhancing capabilities in medical device testing. This move further extends Bureau Veritas' presence and impact in the United States.
- In June 2023, TÜV SÜD has inaugurated a laboratory in New Brighton, Minnesota, with accreditation to ISO 17025 for conducting biological and chemical testing on medical devices. The facility, staffed by proficient scientists and technicians, is designed to serve both domestic and international medical device companies. It will play a pivotal role in supporting research and development efforts by offering services such as microbiology, reusable device testing, chemistry, biocompatibility, and packaging testing.
Answers to Following Key Questions:
- What will be the Medical Device Testing Market’s Trends & growth rate? What analysis has been done of the prices, sales, and volume of the top producers of Medical Device Testing Market?
- What are the main forces behind worldwide Medical Device Testing Market? Which companies dominate Medical Device Testing Market?
- Which companies dominate Medical Device Testing Market? Which business possibilities, dangers, and tactics did they embrace in the market?
- What are the main geographic areas for various trades that are anticipated to have astounding expansion over the Medical Device Testing Market?
- What are the main geographical areas for various industries that are anticipated to observe astounding expansion for Medical Device Testing Market?
- What are the dominant revenue-generating regions for Medical Device Testing Market, as well as regional growth trends?
- By the end of the forecast period, what will the market size and growth rate be?
- What are the main Medical Device Testing Market trends that are influencing the market's expansion?
- Which key product categories dominate Medical Device Testing Market? What is Medical Device Testing Market’s main applications?
- In the coming years, which Medical Device Testing Market technology will dominate the market?
Reason to purchase this Medical Device Testing Market Report:
- Determine prospective investment areas based on a detailed trend analysis of the global Medical Device Testing Market over the next years.
- Gain an in-depth understanding of the underlying factors driving demand for different Medical Device Testing Market segments in the top spending countries across the world and identify the opportunities each offers.
- Strengthen your understanding of the market in terms of demand drivers, industry trends, and the latest technological developments, among others.
- Identify the major channels that are driving the global Medical Device Testing Market, providing a clear picture of future opportunities that can be tapped, resulting in revenue expansion.
- Channelize resources by focusing on the ongoing programs that are being undertaken by the different countries within the global Medical Device Testing Market.
- Make correct business decisions based on a thorough analysis of the total competitive landscape of the sector with detailed profiles of the top Medical Device Testing Market providers worldwide, including information about their products, alliances, recent contract wins, and financial analysis wherever available.
Cloud Engineering Market Size, Share & Trends Analysis, By Deployment (Public, Private, Hybrid), By Service (IaaS, PaaS, SaaS), By Workload, By Enterprise Size By End-use, By Region, And Segment Forecasts
TOC
Table and Figures
Methodology:
At MarketDigits, we take immense pride in our 360° Research Methodology, which serves as the cornerstone of our research process. It represents a rigorous and comprehensive approach that goes beyond traditional methods to provide a holistic understanding of industry dynamics.
This methodology is built upon the integration of all seven research methodologies developed by MarketDigits, a renowned global research and consulting firm. By leveraging the collective strength of these methodologies, we are able to deliver a 360° view of the challenges, trends, and issues impacting your industry.
The first step of our 360° Research Methodology™ involves conducting extensive primary research, which involves gathering first-hand information through interviews, surveys, and interactions with industry experts, key stakeholders, and market participants. This approach enables us to gather valuable insights and perspectives directly from the source.
Secondary research is another crucial component of our methodology. It involves a deep dive into various data sources, including industry reports, market databases, scholarly articles, and regulatory documents. This helps us gather a wide range of information, validate findings, and provide a comprehensive understanding of the industry landscape.
Furthermore, our methodology incorporates technology-based research techniques, such as data mining, text analytics, and predictive modelling, to uncover hidden patterns, correlations, and trends within the data. This data-driven approach enhances the accuracy and reliability of our analysis, enabling us to make informed and actionable recommendations.
In addition, our analysts bring their industry expertise and domain knowledge to bear on the research process. Their deep understanding of market dynamics, emerging trends, and future prospects allows for insightful interpretation of the data and identification of strategic opportunities.
To ensure the highest level of quality and reliability, our research process undergoes rigorous validation and verification. This includes cross-referencing and triangulation of data from multiple sources, as well as peer reviews and expert consultations.
The result of our 360° Research Methodology is a comprehensive and robust research report that empowers you to make well-informed business decisions. It provides a panoramic view of the industry landscape, helping you navigate challenges, seize opportunities, and stay ahead of the competition.
In summary, our 360° Research Methodology is designed to provide you with a deep understanding of your industry by integrating various research techniques, industry expertise, and data-driven analysis. It ensures that every business decision you make is based on a well-triangulated and comprehensive research experience.
• Product Planning Strategy
• New Product Stratergy
• Expanded Research Scope
• Comprehensive Research
• Strategic Consulting
• Provocative and pragmatic
• Accelerate Revenue & Growth
• Evaluate the competitive landscape
• Optimize your partner network
• Analyzing industries
• Mapping trends
• Strategizing growth
• Implementing plans
Covered Key Topics
Growth Opportunities
Market Growth Drivers
Leading Market Players
Company Market Share
Market Size and Growth Rate
Market Trend and Technological
Research Assistance
We will be happy to help you find what you need. Please call us or write to us:
+1 510-730-3200 (USA Number)
Email: sales@marketdigits.com







