- Home
- Healthcare
-
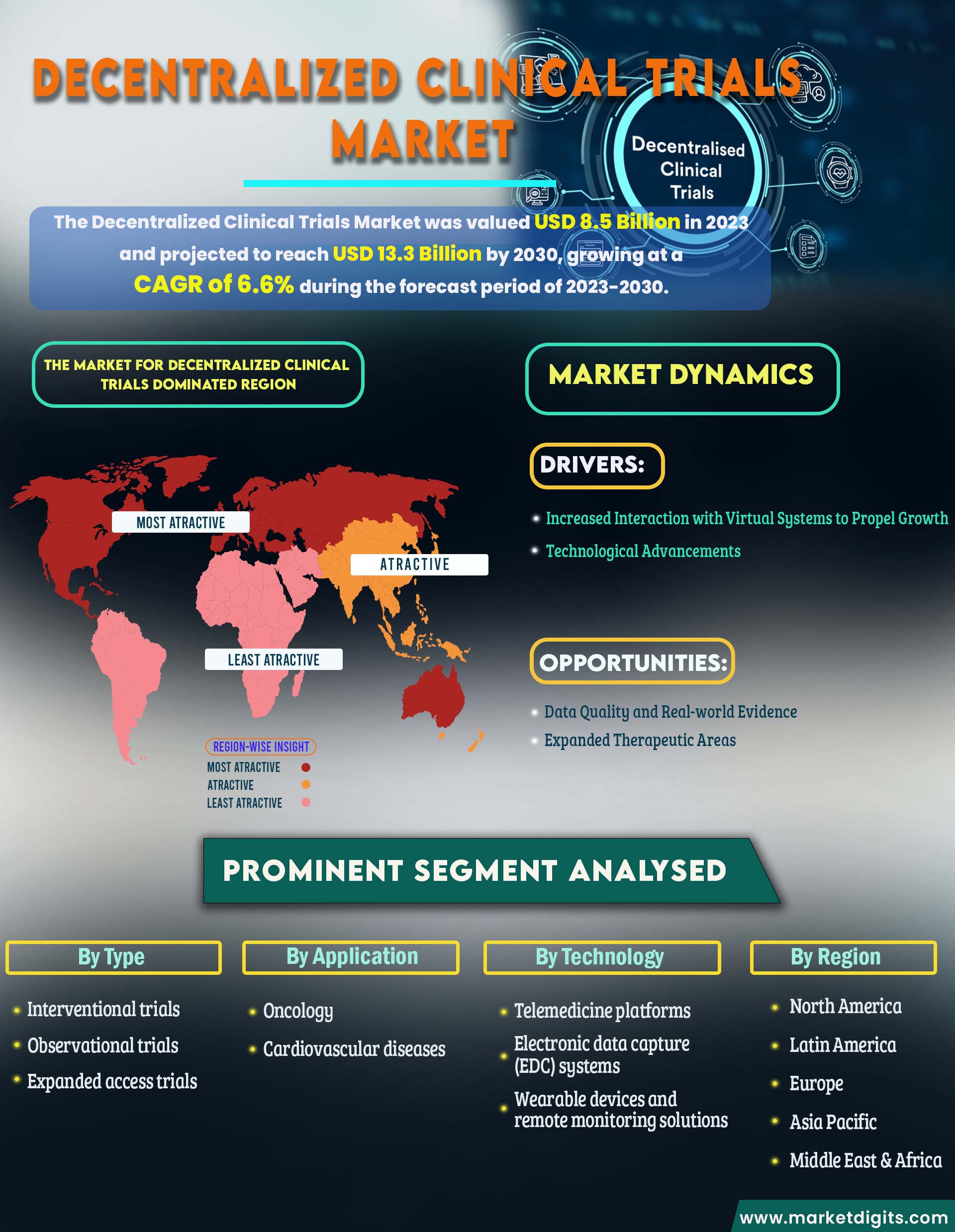
Decentralized Clinical Trials Market

Decentralized Clinical Trials Market by Type (Interventional trials, Observational trials, Expanded access trials), Application , Technology and Region - Partner & Customer Ecosystem (Product Services, Proposition & Key Features) Competitive Index & Regional Footprints by MarketDigits - Forecast 2024-2032
Industry : Healthcare | Pages : 192 Pages | Published On : May 2024
Market Overview
The Decentralized Clinical Trials (DCT) market has experienced significant growth driven by a shift towards patient-centric approaches and technological advancements. DCTs leverage digital health tools, wearable devices, and telehealth solutions to enable remote monitoring, reducing the need for frequent clinic visits. This patient-friendly model enhances inclusivity and diversity in trial participation. The market is characterized by the proliferation of technology providers offering wearables and telehealth platforms, while clinical research organizations play a crucial role in supporting trial conduct. DCTs have gained traction across therapeutic areas, with oncology and neurology at the forefront. As regulatory bodies provide guidance, North America leads in adoption, but global expansion is evident, promising more efficient and inclusive clinical research processes.
Major vendors in the global Decentralized Clinical Trials Market are Clinical Ink, CRF Health, ICON, IQVIA, Labcorp, Medable, Medidata, Oracle, Parexel, PRA Health Sciences, Science 37 and other prominent Players.
Rising concerns related to data privacy
The increased interaction with virtual systems stands out as a prominent driver propelling the growth of the Decentralized Clinical Trials (DCT) market. This driver is closely tied to the integration of digital health technologies, telehealth platforms, and virtual interactions, which collectively enhance the decentralized trial experience. Virtual systems facilitate seamless communication between researchers and participants, enabling remote consultations, eConsent processes, and virtual visits. As the demand for patient-centric trial approaches intensifies, the reliance on virtual systems not only improves participant engagement but also streamlines data collection, ultimately contributing to more efficient and cost-effective clinical trials. This driver reflects the industry's commitment to leveraging technological advancements to create a dynamic and inclusive research environment, where increased interaction with virtual systems plays a pivotal role in driving the growth and success of decentralized clinical trials.
Market Dynamics
Drivers:
- Increased Interaction with Virtual Systems to Propel Growth
- Technological Advancements
Opportunities:
- Data Quality and Real-world Evidence
- Expanded Therapeutic Areas
Decentralized Data Analytics
Decentralized Data Analytics emerges as a pivotal trend in the Decentralized Clinical Trials (DCT) market, reshaping the landscape of clinical research. This trend involves leveraging advanced analytics and artificial intelligence to process and analyze the vast and diverse data generated in decentralized trials. With the integration of digital health technologies and real-time data collection, decentralized trials produce a wealth of information from remote patient monitoring, wearables, and electronic health records. Decentralized Data Analytics not only enhances the speed and efficiency of data processing but also facilitates predictive modeling and actionable insights, contributing to more informed decision-making. By harnessing the power of analytics, researchers can uncover patterns, trends, and correlations in real-world patient data, ultimately improving trial outcomes and accelerating the development of innovative healthcare solutions in a patient-centric and technologically driven clinical research landscape.
The market for Decentralized Clinical Trials is dominated by North America.
North America asserts its dominance as a leading segment in the Decentralized Clinical Trials (DCT) Market. In the United States, the adoption of decentralized approaches is propelled by a robust healthcare infrastructure, technological innovation, and supportive regulatory frameworks from agencies like the FDA. The U.S. pharmaceutical industry's inclination toward patient-centric models further accelerates the integration of DCTs. Canada, as part of the North American landscape, follows suit with a healthcare system that encourages innovation and research. Both nations benefit from a sizable pool of potential trial participants, diverse patient populations, and a strong commitment to advancing clinical research. However, challenges related to regulatory harmonization and data privacy regulations persist, underscoring the need for ongoing collaboration and adaptation to sustain North America's leadership in shaping the future of decentralized clinical trials.
The Interventional trials Segment is Anticipated to Hold the Largest Market Share During the Forecast Period
Based on Type the Decentralized Clinical Trials market is segmented into Interventional trials, Observational trials, Expanded access trials. Interventional trials play a pivotal role in the dominance of the Decentralized Clinical Trials (DCT) market. This segment focuses on actively testing new treatments, drugs, or medical devices, utilizing decentralized approaches that enhance accessibility and patient participation. By incorporating innovative technologies and remote monitoring, interventional trials within the DCT framework streamline data collection, reduce site visits, and enhance patient engagement. The emphasis on real-world evidence, patient-centricity, and the flexibility of decentralized models amplifies the significance of interventional trials, positioning them as a key driver in shaping the landscape of modern clinical research methodologies.
Major Segmentations Are Distributed as follows:
- By Type:
- Interventional trials
- Observational trials
- Expanded access trials
- By Application:
- Oncology
- Cardiovascular diseases
- Others
- By Technology:
- Telemedicine platforms
- Electronic data capture (EDC) systems
- Wearable devices and remote monitoring solutions
- By Region
- North America
- U.S.
- Canada
- Latin America
- Brazil
- Mexico
- Argentina
- Colombia
- Chile
- Peru
- Rest of Latin America
- Europe
- Germany
- France
- Italy
- Spain
- U.K.
- BENELUX
- CIS & Russia
- Nordics
- Austria
- Poland
- Rest of Europe
- Asia Pacific
- North America
-
- China
- Japan
- South Korea
- India
- Thailand
- Indonesia
- Malaysia
- Vietnam
- Australia & New Zealand
- Rest of Asia Pacific
-
- Middle East & Africa
-
- Saudi Arabia
- UAE
- South Africa
- Nigeria
- Egypt
- Israel
- Turkey
- Rest of MEA
Recent Developments
- June 2022, Mediadata, a U.S.-based company and a subsidiary of Dassault Systems Company, launched new clinical operations technology to address critical clinical trial management issues along with Rave CTMS (Clinical Trial Management Systems). It will help both companies to monitor data quickly and visualize it to make quick, enhanced judgments and decisions. This development will improve data oversight and reporting for sponsors and contract research organizations, driving market growth and generating industry growth.
Answers to Following Key Questions:
- What will be the Decentralized Clinical Trials Market’s Trends & growth rate? What analysis has been done of the prices, sales, and volume of the top producers in the Decentralized Clinical Trials Market?
- What are the main forces behind worldwide Decentralized Clinical Trials Market? Which companies dominate Decentralized Clinical Trials Market?
- Which companies dominate Decentralized Clinical Trials Market? Which business possibilities, dangers, and tactics did they embrace in the market?
- What are the global Decentralized Clinical Trials industry's suppliers' opportunities and dangers in Decentralized Clinical Trials Market?
- What is the Decentralized Clinical Trials industry's regional sales, income, and pricing analysis? In the Decentralized Clinical Trials Market, who are the distributors, traders, and resellers?
- What are the main geographic areas for various trades that are anticipated to have astounding expansion over the Decentralized Clinical Trials Market?
- What are the main geographical areas for various industries that are anticipated to observe astounding expansion for Decentralized Clinical Trials Market?
- What are the dominant revenue-generating regions for Decentralized Clinical Trials Market, as well as regional growth trends?
- By the end of the forecast period, what will the market size and growth rate be?
- What are the main Decentralized Clinical Trials Market trends that are influencing the market's expansion?
- Which key product categories dominate Decentralized Clinical Trials Market? What is Decentralized Clinical Trials Market’s main applications?
- In the coming years, which Decentralized Clinical Trials Market technology will dominate the market?
Reason to purchase this Decentralized Clinical Trials Market Report:
- Determine prospective investment areas based on a detailed trend analysis of the global Decentralized Clinical Trials Market over the next years.
- Gain an in-depth understanding of the underlying factors driving demand for different Decentralized Clinical Trials Market segments in the top spending countries across the world and identify the opportunities each offers.
- Strengthen your understanding of the market in terms of demand drivers, industry trends, and the latest technological developments, among others.
- Identify the major channels that are driving the global Decentralized Clinical Trials Market, providing a clear picture of future opportunities that can be tapped, resulting in revenue expansion.
- Channelize resources by focusing on the ongoing programs that are being undertaken by the different countries within the global Decentralized Clinical Trials Market.
- Make correct business decisions based on a thorough analysis of the total competitive landscape of the sector with detailed profiles of the top Decentralized Clinical Trials Market providers worldwide, including information about their products, alliances, recent contract wins, and financial analysis wherever available.
Cloud Engineering Market Size, Share & Trends Analysis, By Deployment (Public, Private, Hybrid), By Service (IaaS, PaaS, SaaS), By Workload, By Enterprise Size By End-use, By Region, And Segment Forecasts
TOC
Table and Figures
Methodology:
At MarketDigits, we take immense pride in our 360° Research Methodology, which serves as the cornerstone of our research process. It represents a rigorous and comprehensive approach that goes beyond traditional methods to provide a holistic understanding of industry dynamics.
This methodology is built upon the integration of all seven research methodologies developed by MarketDigits, a renowned global research and consulting firm. By leveraging the collective strength of these methodologies, we are able to deliver a 360° view of the challenges, trends, and issues impacting your industry.
The first step of our 360° Research Methodology™ involves conducting extensive primary research, which involves gathering first-hand information through interviews, surveys, and interactions with industry experts, key stakeholders, and market participants. This approach enables us to gather valuable insights and perspectives directly from the source.
Secondary research is another crucial component of our methodology. It involves a deep dive into various data sources, including industry reports, market databases, scholarly articles, and regulatory documents. This helps us gather a wide range of information, validate findings, and provide a comprehensive understanding of the industry landscape.
Furthermore, our methodology incorporates technology-based research techniques, such as data mining, text analytics, and predictive modelling, to uncover hidden patterns, correlations, and trends within the data. This data-driven approach enhances the accuracy and reliability of our analysis, enabling us to make informed and actionable recommendations.
In addition, our analysts bring their industry expertise and domain knowledge to bear on the research process. Their deep understanding of market dynamics, emerging trends, and future prospects allows for insightful interpretation of the data and identification of strategic opportunities.
To ensure the highest level of quality and reliability, our research process undergoes rigorous validation and verification. This includes cross-referencing and triangulation of data from multiple sources, as well as peer reviews and expert consultations.
The result of our 360° Research Methodology is a comprehensive and robust research report that empowers you to make well-informed business decisions. It provides a panoramic view of the industry landscape, helping you navigate challenges, seize opportunities, and stay ahead of the competition.
In summary, our 360° Research Methodology is designed to provide you with a deep understanding of your industry by integrating various research techniques, industry expertise, and data-driven analysis. It ensures that every business decision you make is based on a well-triangulated and comprehensive research experience.
• Product Planning Strategy
• New Product Stratergy
• Expanded Research Scope
• Comprehensive Research
• Strategic Consulting
• Provocative and pragmatic
• Accelerate Revenue & Growth
• Evaluate the competitive landscape
• Optimize your partner network
• Analyzing industries
• Mapping trends
• Strategizing growth
• Implementing plans
Covered Key Topics
Growth Opportunities
Market Growth Drivers
Leading Market Players
Company Market Share
Market Size and Growth Rate
Market Trend and Technological
Research Assistance
We will be happy to help you find what you need. Please call us or write to us:
+1 510-730-3200 (USA Number)
Email: sales@marketdigits.com







