- Home
- Healthcare
-
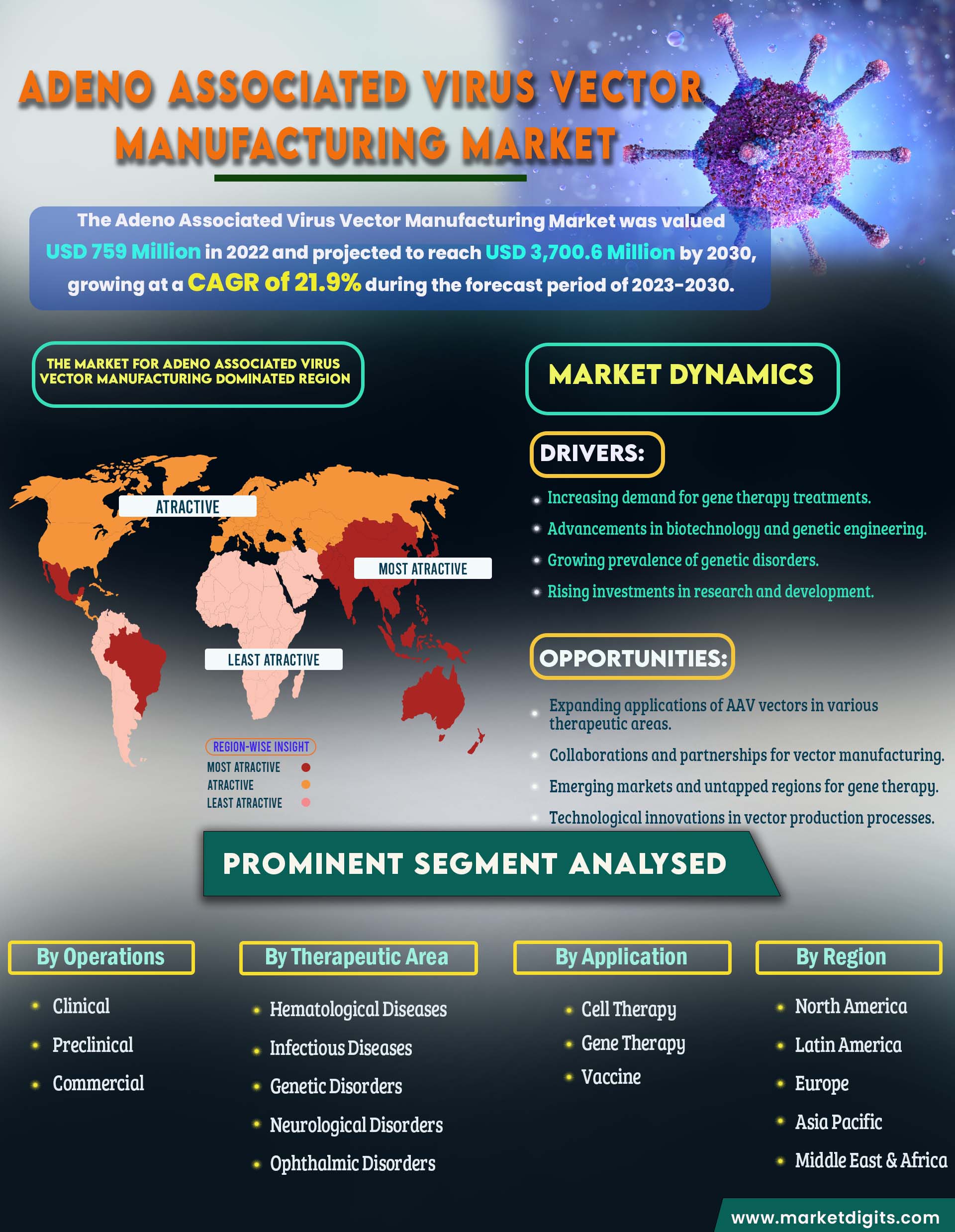
Adeno Associated Virus Vector Manufacturing Market

Adeno Associated Virus Vector Manufacturing Market by Operations (Clinical, Preclinical, Commercial), Therapeutic Area, Application (Cell Therapy, Gene Therapy, Vaccine), Method (In Vitro, In Vivo) and Region - Partner & Customer Ecosystem (Product Services, Proposition & Key Features) Competitive Index & Regional Footprints by MarketDigits - Forecast 2024-2032
Industry : Healthcare | Pages : 191 Pages | Published On : Apr 2024
The global Adeno-Associated Virus (AAV) vector manufacturing market plays a pivotal role in advancing gene therapy and biopharmaceutical research. AAV vectors are essential tools for delivering therapeutic genes into target cells, making them integral in the development of gene therapies for various genetic and acquired diseases. This market's significance lies in its ability to provide scalable and efficient manufacturing solutions for AAV vectors, facilitating the production of gene therapies on a broader scale. As the demand for gene therapies continues to grow, the AAV vector manufacturing market contributes to the overall ecosystem by ensuring a reliable and streamlined supply chain, ultimately benefiting the biopharmaceutical industry in its efforts to bring innovative and transformative therapies to patients.
The market's importance extends beyond facilitating gene therapy development; it also serves as a key enabler for the broader biotechnology and pharmaceutical industry. The consistent growth and advancement of AAV vector manufacturing technologies contribute to the industry's ability to address unmet medical needs and develop novel treatments. In essence, the AAV vector manufacturing market serves as a critical component in the larger ecosystem of biopharmaceutical research and development, supporting the translation of groundbreaking scientific discoveries into tangible therapies that have the potential to revolutionize patient care and outcomes.
Major vendors in the global Adeno Associated Virus Vector Manufacturing Market: Roche, BioMarin Pharmaceutical, Oxford BioMedica, WuXi AppTec, YPOSKESI, Sarepta Therapeutics, Pfizer, Audentes Therapeutics, LifeSpan BioSciences, Inc., GenScript, and Others.
Global Adeno Associated Virus Vector Manufacturing Market Report Scope:
|
Report |
Details |
|
Market size value in 2023 |
USD 759 Million |
|
Market size value in 2030 |
USD 3,700.6 Million |
|
CAGR (2023-2030) |
21.9% |
|
Forecast Period |
2023–2030 |
|
Historic Data |
2019 |
|
Forecast Units |
Value (USD Million/USD Billion) |
|
Segments Covered |
Therapeutic Area, Operations, Application, Method and Region |
|
Geographies Covered |
North America, Europe, Asia Pacific, and RoW |
Increasing Demand for Gene Therapy
The surge in the demand for gene therapy stands as a prominent driver for the global adeno-associated virus (AAV) vector manufacturing market. Gene therapy has gained immense attention as a promising treatment approach for various genetic disorders and chronic diseases. AAV vectors, due to their low immunogenicity and ability to efficiently deliver therapeutic genes into target cells, have become a preferred choice for gene therapy applications. The growing prevalence of genetic disorders, coupled with advancements in molecular biology and genomics, has propelled the need for AAV vectors in gene therapy.
With the approval of several gene therapy products and a robust pipeline of experimental therapies, the demand for AAV vectors is expected to witness substantial growth. Pharmaceutical and biotechnology companies are heavily investing in research and development activities to harness the potential of AAV vectors for treating a wide range of diseases, including inherited genetic disorders, neurodegenerative diseases, and certain types of cancers. As clinical trials continue to demonstrate positive outcomes, the commercialization of gene therapies utilizing AAV vectors is anticipated to escalate, further driving the global market.
Market Dynamics
Drivers:
- Increasing demand for gene therapy treatments.
- Advancements in biotechnology and genetic engineering.
- Growing prevalence of genetic disorders.
- Rising investments in research and development.
Opportunities:
- Expanding applications of AAV vectors in various therapeutic areas.
- Collaborations and partnerships for vector manufacturing.
- Emerging markets and untapped regions for gene therapy.
- Technological innovations in vector production processes.
Technological Advancements in AAV Vector Manufacturing
Technological advancements in AAV vector manufacturing processes have emerged as a crucial driver for market growth. The production of AAV vectors involves complex and sophisticated bioprocessing techniques, including cell culture, purification, and formulation. Continuous efforts to enhance the efficiency, scalability, and cost-effectiveness of AAV vector manufacturing have resulted in the development of novel technologies and platforms.
Innovations such as single-use bioreactors, improved cell lines, and optimized purification methods have contributed to streamlining the manufacturing process and increasing the yield of high-quality AAV vectors. These advancements not only address the scalability challenges associated with large-scale production but also facilitate the development of AAV-based gene therapies for a broader patient population. As manufacturing technologies continue to evolve, the industry is poised to overcome existing limitations and meet the escalating demand for AAV vectors in gene therapy applications.
North America dominates the market for Adeno Associated Virus Vector Manufacturing.
The dominating region in the global adeno-associated virus vector manufacturing market is North America, with the United States leading the way. The region's dominance is attributed to the presence of a well-established biotechnology and pharmaceutical sector, strong research and development infrastructure, and a supportive regulatory environment for gene therapy products. In the United States, major biopharmaceutical companies and academic institutions are actively engaged in AAV vector manufacturing for various gene therapy applications. The U.S. Food and Drug Administration (FDA) has played a pivotal role in fostering the development and approval of gene therapies, providing a conducive environment for market growth.
While North America currently dominates the market, Asia-Pacific is emerging as a region with high growth potential. Countries like China and Japan are investing significantly in biotechnology and gene therapy research. The rising prevalence of genetic disorders, coupled with favorable government initiatives, is driving the growth of the AAV vector manufacturing market in the Asia-Pacific region. Additionally, the comparatively lower manufacturing costs in some Asian countries contribute to their attractiveness as potential hubs for AAV vector production. As the regulatory landscape evolves and infrastructure strengthens, Asia-Pacific is poised to become a key player in the global AAV vector manufacturing market.
The Operations Segments is anticipated to hold the Largest Market Share during the Forecast Period
The Global Adeno-Associated Virus (AAV) Vector Manufacturing Market comprises three key segments for the operations segment: clinical, preclinical, and commercial, each representing different stages in the development and deployment of AAV vectors. The Clinical segment, focusing on therapeutic applications and trials, dominates the scale of operations due to the increasing demand for gene therapies across various medical conditions. As the field of gene therapy advances, more clinical trials are initiated to assess the efficacy and safety of AAV vectors in treating diseases. The Preclinical segment plays a crucial role in the early stages of research and development, contributing to the pipeline of potential therapies. However, the Commercial segment takes the lead as gene therapies receive regulatory approvals and move towards mass production and distribution, reflecting the growing commercialization of AAV vector-based treatments. The dominance of the Clinical segment underscores the pivotal role of AAV vectors in advancing gene therapies through rigorous testing and validation in real-world patient populations.
Major Segmentations Are Distributed as follows:
- By Operations
- Clinical
- Preclinical
- Commercial
- By Therapeutic Area
- Hematological Diseases
- Infectious Diseases
- Genetic Disorders
- Neurological Disorders
- Ophthalmic Disorders
- Others
- By Application
- Cell Therapy
- Gene Therapy
- Vaccine
- By Method
- In Vitro
- In Vivo
- By Region
- North America
- US
- Canada
- Latin America
- Brazil
- Mexico
- Argentina
- Colombia
- Chile
- Peru
- Rest of Latin America
- Europe
- UK
- Germany
- France
- Italy
- Spain
- Russia
- BENELUX
- CIS & Russia
- Nordics
- Austria
- Poland
- Rest of Europe
- Asia Pacific
- China
- Japan
- India
- South Korea
- Thailand
- Indonesia
- Malaysia
- Vietnam
- Australia & New Zealand
- Rest of Asia Pacific
- Middle East & Africa
- Saudi Arabia
- UAE
- South Africa
- Nigeria
- Egypt
- Israel
- Turkey
- Rest of Middle East & Africa
- North America
Recent Developments
- In June 2022, to produce AAV gene therapies based on NAV Technology on a 2000L scale, REGENXBIO Inc. developed an AAV vector-based gene therapy facility. This cutting-edge facility in Maryland was opened by the corporation with an investment of $65 million.
Adeno Associated Virus Vector Manufacturing Market Report Gives Answers to Following Key Questions:
- What will be the Adeno Associated Virus Vector Manufacturing Market’s Trends & growth rate? What analysis has been done of the prices, sales, and volume of the top producers of Adeno Associated Virus Vector Manufacturing Market?
- What are the main forces behind the worldwide Adeno Associated Virus Vector Manufacturing Market? Which companies dominate the Adeno Associated Virus Vector Manufacturing Market?
- Which companies dominate the Adeno Associated Virus Vector Manufacturing Market? Which business possibilities, dangers, and tactics did they embrace in the market?
- What are the global Adeno Associated Virus Vector Manufacturing industry's suppliers' opportunities and dangers in Adeno Associated Virus Vector Manufacturing Market?
- What is the Adeno Associated Virus Vector Manufacturing industry's regional sales, income, and pricing analysis? In the Adeno Associated Virus Vector Manufacturing Market, who are the distributors, traders, and resellers?
- What are the main geographic areas for various trades that are anticipated to have astounding expansion over the Adeno Associated Virus Vector Manufacturing Market?
- What are the main geographical areas for various industries that are anticipated to observe an astounding expansion in Adeno Associated Virus Vector Manufacturing Market?
- What are the dominant revenue-generating regions for Adeno Associated Virus Vector Manufacturing Market, as well as regional growth trends?
- By the end of the forecast period, what will the market size and growth rate be?
- What are the main Adeno Associated Virus Vector Manufacturing Market trends that are influencing the market's expansion?
- Which key product categories dominate the Adeno Associated Virus Vector Manufacturing Market? What are the Adeno Associated Virus Vector Manufacturing Market’s main applications?
- In the coming years, which Adeno Associated Virus Vector Manufacturing Market technology will dominate the market?
Reason to purchase this Adeno Associated Virus Vector Manufacturing Market Report:
- Determine prospective investment areas based on a detailed trend analysis of the global Adeno Associated Virus Vector Manufacturing Market over the next years.
- Gain an in-depth understanding of the underlying factors driving demand for different Adeno Associated Virus Vector Manufacturing Market segments in the top spending countries across the world and identify the opportunities each offers.
- Strengthen your understanding of the market in terms of demand drivers, industry trends, and the latest technological developments, among others.
- Identify the major channels that are driving the global No Associated Virus Vector Manufacturing Market, providing a clear picture of future opportunities that can be tapped, resulting in revenue expansion.
- Channelize resources by focusing on the ongoing programs that are being undertaken by the different countries within the global No Associated Virus Vector Manufacturing Market.
- Make correct business decisions based on a thorough analysis of the total competitive landscape of the sector with detailed profiles of the top Adeno Associated Virus Vector Manufacturing Market providers worldwide, including information about their products, alliances, recent contract wins, and financial analysis wherever available.
Cloud Engineering Market Size, Share & Trends Analysis, By Deployment (Public, Private, Hybrid), By Service (IaaS, PaaS, SaaS), By Workload, By Enterprise Size By End-use, By Region, And Segment Forecasts
TOC
Table and Figures
Methodology:
At MarketDigits, we take immense pride in our 360° Research Methodology, which serves as the cornerstone of our research process. It represents a rigorous and comprehensive approach that goes beyond traditional methods to provide a holistic understanding of industry dynamics.
This methodology is built upon the integration of all seven research methodologies developed by MarketDigits, a renowned global research and consulting firm. By leveraging the collective strength of these methodologies, we are able to deliver a 360° view of the challenges, trends, and issues impacting your industry.
The first step of our 360° Research Methodology™ involves conducting extensive primary research, which involves gathering first-hand information through interviews, surveys, and interactions with industry experts, key stakeholders, and market participants. This approach enables us to gather valuable insights and perspectives directly from the source.
Secondary research is another crucial component of our methodology. It involves a deep dive into various data sources, including industry reports, market databases, scholarly articles, and regulatory documents. This helps us gather a wide range of information, validate findings, and provide a comprehensive understanding of the industry landscape.
Furthermore, our methodology incorporates technology-based research techniques, such as data mining, text analytics, and predictive modelling, to uncover hidden patterns, correlations, and trends within the data. This data-driven approach enhances the accuracy and reliability of our analysis, enabling us to make informed and actionable recommendations.
In addition, our analysts bring their industry expertise and domain knowledge to bear on the research process. Their deep understanding of market dynamics, emerging trends, and future prospects allows for insightful interpretation of the data and identification of strategic opportunities.
To ensure the highest level of quality and reliability, our research process undergoes rigorous validation and verification. This includes cross-referencing and triangulation of data from multiple sources, as well as peer reviews and expert consultations.
The result of our 360° Research Methodology is a comprehensive and robust research report that empowers you to make well-informed business decisions. It provides a panoramic view of the industry landscape, helping you navigate challenges, seize opportunities, and stay ahead of the competition.
In summary, our 360° Research Methodology is designed to provide you with a deep understanding of your industry by integrating various research techniques, industry expertise, and data-driven analysis. It ensures that every business decision you make is based on a well-triangulated and comprehensive research experience.
• Product Planning Strategy
• New Product Stratergy
• Expanded Research Scope
• Comprehensive Research
• Strategic Consulting
• Provocative and pragmatic
• Accelerate Revenue & Growth
• Evaluate the competitive landscape
• Optimize your partner network
• Analyzing industries
• Mapping trends
• Strategizing growth
• Implementing plans
Covered Key Topics
Growth Opportunities
Market Growth Drivers
Leading Market Players
Company Market Share
Market Size and Growth Rate
Market Trend and Technological
Research Assistance
We will be happy to help you find what you need. Please call us or write to us:
+1 510-730-3200 (USA Number)
Email: sales@marketdigits.com







